Which metals are in smartphones?¶
Authors and date
- Submitted on: June 6th 2021
- Françoise Berthoud: research engineer in computer science at CNRS, EcoInfo
This sheet, automatically translated by the Deepl tool, has not been proofread by its author
Metals in smartphones, for what purpose 1?¶
In 1950, we counted a dozen metals in our good old landline phones. In the 1990s, mobile phones, far from fitting in our pockets, contained a little over thirty metals. Today's smartphone, much smaller and thinner, contains more than 50 metals: a condition to have all the features of our equipment!
A few examples:
Indium and tin are needed to manufacture our screens to transform the touch of our index finger into a "click". Indeed, these so-called capacitive screens are coated with a film that conducts electricity while being transparent and it is an indium-tin oxide that allows this technological feat.
As for LEDs, they contain a compound of gallium (extracted from aluminum ore) and at least one other atom, which will determine the "basic" color of the LED. Thus, arsenic combined with phosphorus will give an orange-red light, while with nitrogen or indium, the LED appears blue. But the designers of LEDs have imagined being able to make more beautiful and varied colors, yellow, white, red, blue or green. Then are used different cocktails of rare earths with mysterious names: yttrium garnet and aluminum, cerium, yttrium, europium or terbium. We can also find lanthanum and gadolinium.
Focus on Rare Earths
Improperly called rare earths, lanthanides are actually as abundant as other metals like nickel or copper and therefore more abundant than gold and silver but much more dispersed: they are called cerium, europium, gadolinium, lanthanum, neodymium, praseodymium, promethium, samarium and scandium, dysprosium, terbium, holmium, lutetium, terbium, thulium, ytterbium and yttrium.
These metals are found in trace amounts in most natural environments. Their electronic, magnetic, optical or catalytic properties make them particularly sought after by industry (aeronautics, automobile, technologies for people with disabilities, etc.).
The methods of extraction and separation of these metals require complex and highly polluting processes: discharge of acids, bases, solvents, heavy metals or radioactive waste2. In addition, these processes require large quantities of water.
Rare earth elements (REE) are used in many industrial applications: electronics, clean energy, aerospace, automotive, defense and others.
For example, magnet manufacturing accounted for the largest and most important end use of REEs in 2019, accounting for 38% of the forecast demand. Magnets are used in smartphones, televisions, computers, automobiles, wind turbines, jet aircraft and many other products3.
Rare earths are also found in magnets that are needed to produce the vibrations in our smartphones: neodymium, praseodymium, terbium and dysprosium. As for tungsten, which is twice as heavy as steel, it is used as a weight to amplify the vibrations.
Most of the metal weight in smartphones is not made up of rare earths. Lithium and cobalt, for example, are found in lithium-ion batteries and a host of metals of varying degrees of rarity and preciousness in integrated circuits and printed circuit boards. Some details:
-
An integrated circuit is a plate of semiconductor material (usually silicon, sometimes germanium) to the surface of which transistors, small three-legged components also containing semiconductor material, are connected. This is also called a chip. In order to make the silicon electrically conductive in certain areas, it must be "doped" with impurities such as phosphorus, boron, arsenic, antimony, but also indium or gallium. In transistors that must operate at very high frequencies, such as those used for Wi-Fi, Bluetooth or 4G, silicon is replaced by gallium arsenide or silicon germanium.
-
With the launch of its 45nm generation in 2007, Intel began using4 hafnium to insulate the gates of its transistors. These are connected together on the circuit boards by titanium and tungsten films. The miniaturization of chips would push semiconductor manufacturers to replace copper, which is increasingly impractical at the nanoscale, with cobalt or ruthenium.
-
As for printed circuits, they are epoxy resin plates, often green in color, on which components are soldered thanks to copper tracks. An excellent electrical conductor, gold is also found in printed circuits, constituting in particular the connecting wires between the silicon and the pins of the various components. Silver is present in most resistors. Very small quantities of palladium are also found in printed circuits.
-
Finally, one of the most common components soldered to circuit boards is the capacitor, a small tank of electrical energy. Standard capacitors use aluminum, but these are too big to fit in a smartphone. To make very small ones, tantalum is used to make so-called "tantalum drop" capacitors, so called because of their easily recognizable shape. The physical properties of this metal (notably a very high melting point of 3 016, 85 °C) make it a material of choice for this type of application.
What quantities?¶
To put things in perspective in relation to the total weight of a smartphone, you should know that, depending on the model:
- plastics and synthetics account for 30 to 50% of the materials ;
- glass and ceramics represent 10 to 20%;
- metals account for 40 to 60% of the materials in a smartphone.
Of this amount of metals:
- 80 to 85% are ferrous and non-ferrous materials such as copper, aluminum, zinc, tin, chrome or nickel ...
- 0.5% are precious metals: gold, silver, platinum, palladium...
- And 0.1% of rare earth and special metals: europium, yttrium, terbium, gallium, tungsten, indium, etc.
What represents in practice for a classic smartphone:
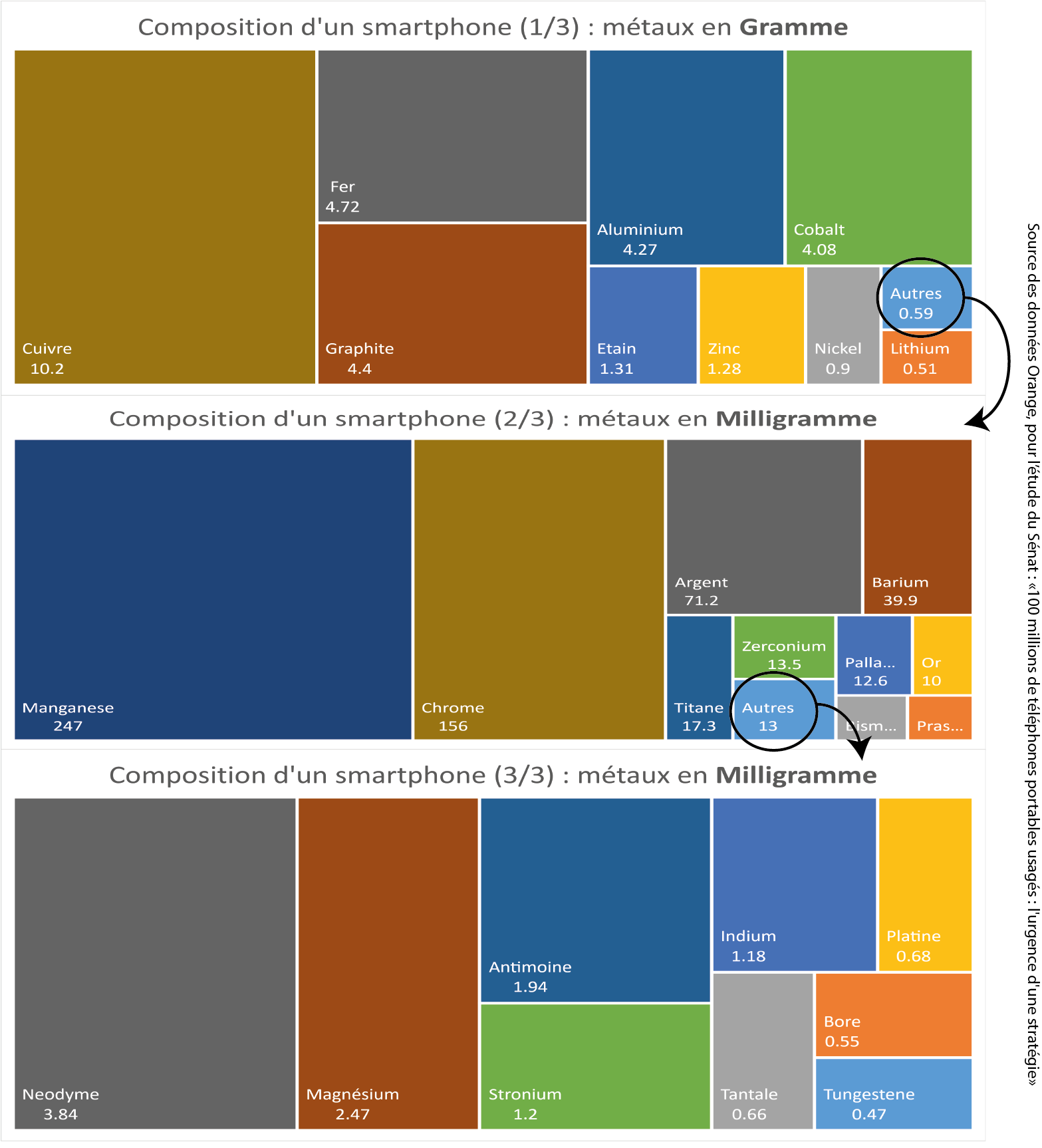 Composition of a classic smartphone, source Orange data for a Senate study5
Composition of a classic smartphone, source Orange data for a Senate study5
Globally and relative to the total volume of metals produced for all sectors, the needs for digital technologies do not represent a large proportion, except for certain metals whose volume produced is low compared to the major strategic metals such as Tantalum, Indium, Germanium, and certain rare earths, but which are highly strategic.
To go further:¶
Liliane Dedryver, with the help of Vincent Couric. La consommation de métaux du numérique : un secteur loin d’être dématérialisé [online]. FRANCE STRATÉGIE, Working Paper n°2020-05, 06/2020. Available at strategie.gouv [21/06/2021]
World Mining Data. site [06/21/2021]
Le smartphone, pas si « smart » pour l’environnement [online]. ADEME, 09/2020, maj 11/2020. Available at ADEME [21/06/2021]
Sources¶
-
Most of this information is taken from the the french site https://www.frandroid.com/comment-faire/comment-fonctionne-la-technologie/613459_a-quoi-servent-les-metaux-rares-dans-nos-smartphones ↩
-
Michel Latroche. Les terres rares, et après ?. CNRS Le journal, 05/2019. Disponible sur lejournal.cnrs.fr [21/06/2021] ↩
-
Faits sur les éléments des terres rares. Disponible sur www.rncan.gc.ca. Modified on February 2021. ↩
-
Intel's Fundamental Advance in Transistor Design Extends Moore's Law, Computing Performance. SANTA CLARA, Calif, Nov. 11, 2007. Available at intel.com ↩